Electron configuration chart aluminum images are ready in this website. Electron configuration chart aluminum are a topic that is being searched for and liked by netizens today. You can Find and Download the Electron configuration chart aluminum files here. Find and Download all free images.
If you’re looking for electron configuration chart aluminum images information related to the electron configuration chart aluminum keyword, you have pay a visit to the ideal site. Our website always gives you hints for seeing the maximum quality video and image content, please kindly hunt and find more enlightening video content and images that match your interests.
What electron configuration matches an oxygen atom? A shorthand way to write the electron configuration, called noble gas notation, is [ne]3^23p^1. 2 8 3 aluminum discovery. An electron configuration can quickly and simply tell a reader how many electron orbitals an atom has as well as the number of electrons populating each of its orbitals. The basis of this prediction is a rule known as the aufbau principle , which assumes that electrons are added to an atom, one at a time, starting with the lowest energy orbital, until all of the electrons have been placed in an appropriate orbital.
Electron Configuration Chart Aluminum. In this lecture we continue the discussion of quantum numbers and their use in electron configurations as well as the relationship of electron configuration to the periodic properties of the elements. As an approximate rule, electron configurations are given by the aufbau principle and the madelung rule. Shortly after, however, the international union of pure and applied chemistry (or iupac) stepped in, standardizing the suffix to the more conventional �ium�. What is the electron configuration and orbital diagram of:
 Unique Periodic Table Groups Properties Pdf Periodic From pinterest.com
Unique Periodic Table Groups Properties Pdf Periodic From pinterest.com
A neutral atom of aluminum has 13 protons and 13 electrons. An electron configuration can quickly and simply tell a reader how many electron orbitals an atom has as well as the number of electrons populating each of its orbitals. [ne]3s 2 3p 1 long form: It was used in tanning, dyeing, and as an aid to stop minor bleeding and even as an ingredient in baking powder. In this lecture we continue the discussion of quantum numbers and their use in electron configurations as well as the relationship of electron configuration to the periodic properties of the elements. An orbital is defined as the most probable location for finding an electron.
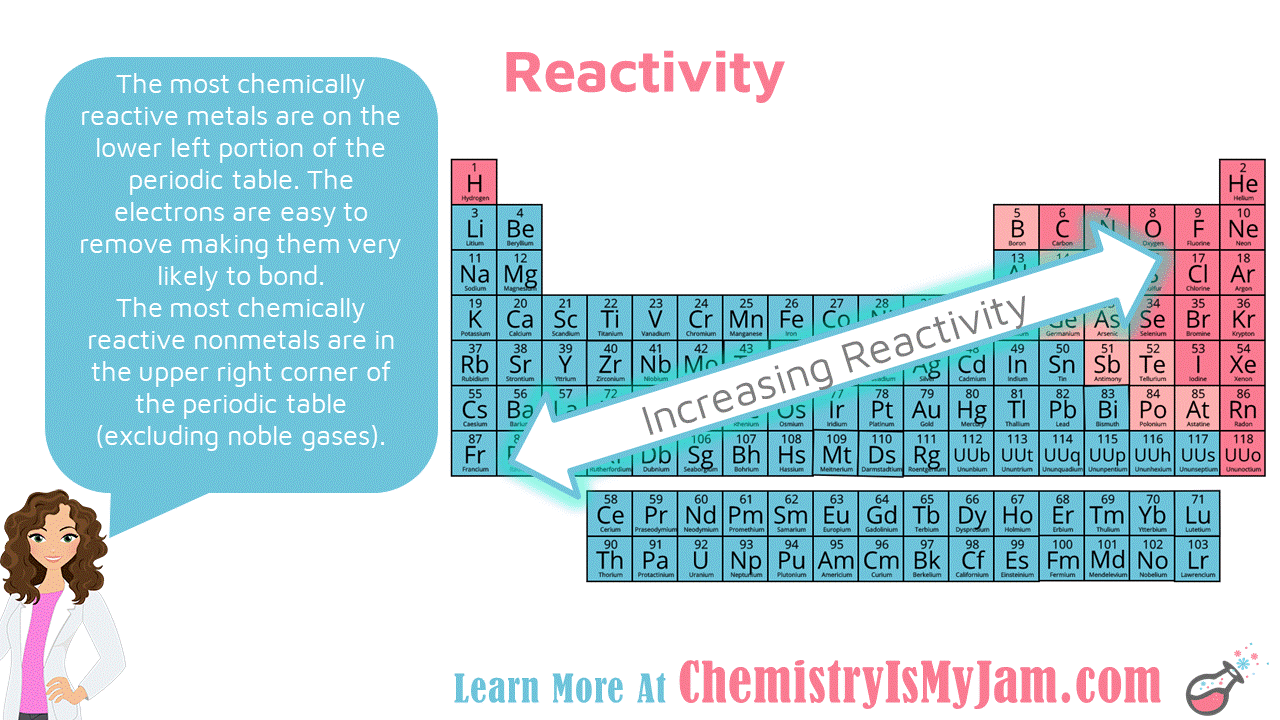
This diagram shows the electron configuration of aluminum.
29 and should be [ar]3d 9 2s 2, but it has been to be determined to be [ar]3d 10 4s 1. The basis of this prediction is a rule known as the aufbau principle , which assumes that electrons are added to an atom, one at a time, starting with the lowest energy orbital, until all of the electrons have been placed in an appropriate orbital. First, write out the electron configuration for each parent atom. Each orbital holds 2 electrons. For example, krypton ends the 4th row so you would begin with 5. 1s 2 2s 2 2p 6 3s 2.
 Source: pinterest.com
Source: pinterest.com
Its electron configuration is (1s^2 2s^2 2p^6 3s^1). Electron configuration [ne] 3s 2 3p 1 cas number: In this lecture we continue the discussion of quantum numbers and their use in electron configurations as well as the relationship of electron configuration to the periodic properties of the elements. The ground state electron configuration for aluminum is 1s^22s^22p^63s^23p^1. 1s 2 2s 2 2p 6 given :
 Source: pinterest.com
Source: pinterest.com
The basis of this prediction is a rule known as the aufbau principle , which assumes that electrons are added to an atom, one at a time, starting with the lowest energy orbital, until all of the electrons have been placed in an appropriate orbital. The ground state electron configuration for aluminum is 1s^22s^22p^63s^23p^1. Iron is a unique element , which is around and inside us. An atom�s electron configuration is a numeric representation of its electron orbitals. 1s 2 2s 2 p 6 3s 2 p 1;
 Source: pinterest.com
Source: pinterest.com
What is the electron configuration and orbital diagram of: 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2. 2 8 3 aluminum discovery. Each orbital holds 2 electrons. The electron configuration for calcium is:
 Source: pinterest.com
Source: pinterest.com
Sublevels can be broken down into regions called orbitals. The electron configuration for aluminum is 1s2 2p2 3s6 3p1. For example, krypton ends the 4th row so you would begin with 5. Electron configuration [ne] 3s 2 3p 1 cas number: In this lecture we continue the discussion of quantum numbers and their use in electron configurations as well as the relationship of electron configuration to the periodic properties of the elements.
 Source: pinterest.com
Source: pinterest.com
For example, the electron configuration of sodium is 1s 2 2s 2 2p 6 3s 1. Continue your electron configuration using the row after the noble gas. Value is a guess based on periodic table trend. 1s 2 2s 2 2p 6 3s 2 3p 1 shell structure: It is called the box and arrow (or circle and x) orbital configuration.
 Source: pinterest.com
Source: pinterest.com
Value is a guess based on periodic table trend. Value is a guess based on periodic table trend. Continue writing your electron configuration following the chart until you reach the correct number of electrons. This diagram shows the electron configuration of aluminum. There is yet another way to writing electron configurations.
 Source: pinterest.com
Source: pinterest.com
1s 2 2s 2 p 6 3s 2 p 1; 1s 2 2s 2 2p 6 3s 2 3p 1 shell structure: 29 and should be [ar]3d 9 2s 2, but it has been to be determined to be [ar]3d 10 4s 1. 1s 2 2s 2 2p 6 given : So although a neutral atom of aluminum has 13 electrons, the ion of aluminum, al 3+, has lost three electrons and only has 10.
 Source: pinterest.com
Source: pinterest.com
It is called the box and arrow (or circle and x) orbital configuration. Its electron configuration is (1s^2 2s^2 2p^6 3s^1). Iron is a unique element , which is around and inside us. The electron configuration for aluminum is 1s2 2p2 3s6 3p1. What atom matches this electron configuration?
 Source: pinterest.com
Source: pinterest.com
Electron configuration [ne] 3s 2 3p 1 cas number: Value is a guess based on periodic table trend. So although a neutral atom of aluminum has 13 electrons, the ion of aluminum, al 3+, has lost three electrons and only has 10. An orbital is defined as the most probable location for finding an electron. What atom matches this electron configuration?
 Source: in.pinterest.com
Source: in.pinterest.com
There are 118 elements in the periodic table. Iron is a unique element , which is around and inside us. Aluminum will lose three electrons when it forms an ion. [ne]3s 2 3p 1 long form: For example, krypton ends the 4th row so you would begin with 5.
 Source: pinterest.com
Source: pinterest.com
29 and should be [ar]3d 9 2s 2, but it has been to be determined to be [ar]3d 10 4s 1. Notes on the electron configuration of particular elements: 1s 2 2s 2 2p 6 given : A shorthand way to write the electron configuration, called noble gas notation, is [ne]3^23p^1. Each element has a unique atomic structure that is influenced by its electronic configuration, which is the distribution of electrons across different orbitals of an atom.
This site is an open community for users to do submittion their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site value, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title electron configuration chart aluminum by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.





